IG: the standard for PIDD therapy2
IG is a purified plasma product with antibodies made by the body’s immune system.

IG is a purified plasma product with antibodies made by the body’s immune system.
IG is used to treat a spectrum of immune disorders, including6*:
Immunodeficiency disorders
Primary immunodeficiency disease (PIDD)
Autoimmune disorders
Chronic inflammatory demyelinating polyneuropathy (CIDP)†
Immune thrombocytopenia (ITP)†
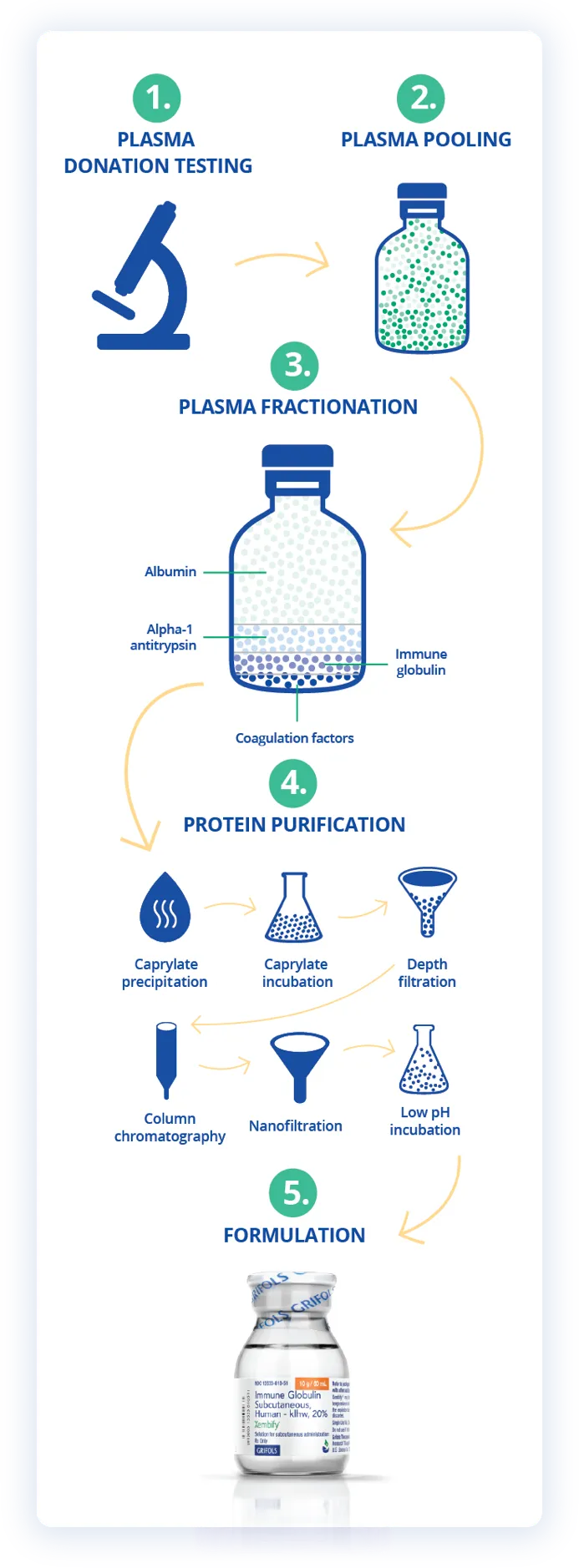

Proven SCIG and IVIG treatments to meet the needs of your patients with PIDD5,7,8,10-13
Concentrations
20%
10%
Routes of Administration and Indications
Subcutaneous
PIDD in patients 2 years of age and older
FDA Approval: July 3, 2019
Intravenous
PIDD in patients 2 years of age and older, idiopathic thrombocytopenic purpura (ITP) in adults and children, and chronic inflammatory demyelinating polyneuropathy (CIDP) in adults
FDA Approval: August 27, 2003 (PIDD and ITP); September 12, 2008 (CIDP)
Subcutaneous
PIDD in patients 2 years of age and older
FDA Approval: October 13, 2010
Gamma Globulin Content (%)
100
100
Monomers (%)
99±1 monomers + dimers
100 monomers + dimers
IgG Content (%)
≥ 98
≥ 98
IgA Content
≤ 0.07 mg/mL
0.046 mg/mL
IgM Content
< 0.004 mg/mL
< 0.005 mg/mL
pH
4.1-4.8
4.0-4.5
Sodium Content
Trace
Trace (< 7 mEq/L)
Sugar Content
Sugar-free
Sugar-free
Stabilizer and Tonicity Modifier
Glycine
Glycine
| Product Details |
XEMBIFY (immune globulin subcutaneous human–klhw) 20% |
GAMUNEX-C (immune globulin injection [human], 10% caprylate/chromatography purified) |
|---|---|---|
|
Concentrations |
20% |
10% |
|
Routes of Administration and Indications |
Subcutaneous FDA Approval: July 3, 2019 |
Intravenous Subcutaneous |
|
Gamma Globulin Content (%) |
100 |
100 |
|
Monomers (%) |
99±1 monomers + dimers |
100 monomers + dimers |
|
IgG Content (%) |
≥ 98 |
≥ 98 |
|
IgA Content |
≤ 0.07 mg/mL |
0.046 mg/mL |
|
IgM Content |
< 0.004 mg/mL |
< 0.005 mg/mL |
|
pH |
4.1-4.8 |
4.0-4.5 |
|
Sodium Content |
Trace |
Trace (< 7 mEq/L) |
|
Sugar Content |
Sugar-free |
Sugar-free |
|
Stabilizer and Tonicity Modifier |
Glycine |
Glycine |
Factors to consider when choosing between IVIG and SCIG8,14
Patient dexterity
Not important
Important
Compliance
Healthcare professional dependent
Patient dependent
Independence
Rely on nurse or center
Rely on self
Portability
Limited
Yes
School or work absence
Likely, if at infusion suite
Less likely, if at home
Length of infusion
1-4 hours
~1 hour
Frequency
Every 3-4 weeks
Flexible: daily, up to biweekly
Location
Home or infusion suite
Home usually
Home nurse (for initial treatment)
Yes
Yes
Ongoing nurse support
Yes
Partial support
Number of needle sticks per infusion
1
1 or more
Pharmacokinetics
Peaks/troughs
Steady state
| Factors | IVIG | SCIG |
|---|---|---|
|
Patient dexterity |
Not important |
Important |
|
Compliance |
Healthcare professional dependent |
Patient dependent |
|
Independence |
Rely on nurse or center |
Rely on self |
|
Portability |
Limited |
Yes |
|
School or work absence |
Likely, if at infusion suite |
Less likely, if at home |
|
Length of infusion |
1-4 hours |
~1 hour |
|
Frequency |
Every 3-4 weeks |
Flexible: daily, up to biweekly |
|
Location |
Home or infusion suite |
Home usually |
|
Home nurse (for initial treatment) |
Yes |
Yes |
|
Ongoing nurse support |
Yes |
Partial support |
|
Number of needle sticks per infusion |
1 |
1 or more |
|
Pharmacokinetics |
Peaks/troughs |
Steady state |
Contraindications
GAMUNEX-C and XEMBIFY are contraindicated in patients who have had an anaphylactic or severe systemic reaction to the administration of human immune globulin and in IgA-deficient patients with antibodies against IgA and a history of hypersensitivity.
You and your patient with PIDD can decide together whether XEMBIFY or GAMUNEX-C is right for them
*The disorders shown above do not include all indications for IG therapy.
†XEMBIFY is not indicated for CIDP or ITP.8
‡The average IgA content is ≤ 0.07 mg/mL and the average IgM content is <0.004 mg/mL.7
Indication
XEMBIFY® (immune globulin subcutaneous human–klhw) is a 20% immune globulin indicated for treatment of primary humoral immunodeficiency disease (PIDD) in patients 2 years of age and older. XEMBIFY is for subcutaneous administration only.
Important Safety Information
WARNING: THROMBOSIS
Contraindications
XEMBIFY is contraindicated in patients who have had an anaphylactic or severe systemic reaction to the administration of human immune globulin. It is contraindicated in IgA-deficient patients with antibodies against IgA and a history of hypersensitivity.
Warnings and Precautions
Aseptic meningitis syndrome (AMS). AMS may occur with human immune globulin treatment, including XEMBIFY. Conduct a thorough neurological exam on patients exhibiting signs and symptoms to rule out other causes of meningitis. Discontinuation of treatment has resulted in remission within several days without sequelae.
Thrombosis. Thrombosis may occur following treatment with immune globulin products, including XEMBIFY. Thrombosis may occur in the absence of known risk factors. In patients at risk, administer at the minimum dose and infusion rate practicable. Ensure adequate hydration before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk of hyperviscosity.
Hypersensitivity. Severe hypersensitivity reactions may occur with immune globulin products, including XEMBIFY. In case of hypersensitivity, discontinue infusion immediately and institute appropriate treatment. XEMBIFY contains IgA. Patients with known antibodies to IgA may have a greater risk of developing potentially severe hypersensitivity and anaphylactic reactions.
Renal dysfunction/failure. Acute renal dysfunction/failure, acute tubular necrosis, proximal tubular nephropathy, osmotic nephrosis, and death may occur with use of human immune globulin products, especially those containing sucrose. XEMBIFY does not contain sucrose. Ensure patients are not volume-depleted prior to starting infusion. In patients at risk due to preexisting renal insufficiency or predisposition to acute renal failure, assess renal function prior to the initial infusion of XEMBIFY and again at appropriate intervals thereafter. If renal function deteriorates, consider discontinuation.
Hemolysis. XEMBIFY may contain blood group antibodies that may cause a positive direct antiglobulin reaction and hemolysis. Monitor patients for clinical signs and symptoms of hemolysis. If signs and symptoms are present after infusion, perform confirmatory lab testing.
Transfusion-related acute lung injury (TRALI). Noncardiogenic pulmonary edema may occur in patients following treatment with immune globulin products, including XEMBIFY. Monitor patients for pulmonary adverse reactions. If TRALI is suspected, perform appropriate tests for the presence of antineutrophil and anti-HLA antibodies in both the product and patient serum. TRALI may be managed using oxygen therapy with adequate ventilatory support.
Transmissible infectious agents. Because XEMBIFY is made from human blood, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. No cases of transmission of viral diseases, vCJD, or CJD have ever been associated with the use of XEMBIFY.
Interference with lab tests. After infusion of XEMBIFY, passively transferred antibodies in the patient’s blood may yield positive serological testing results, with the potential for misleading interpretation.
Adverse Reactions
The most common adverse reactions in ≥ 5% of subjects in the clinical trial were local adverse reactions, including infusion-site erythema (redness), infusion-site pain, infusion-site swelling (puffiness), infusion-site bruising, infusion-site nodule, infusion-site pruritus (itching), infusion-site induration (firmness), infusion-site scab, infusion-site edema, and systemic reactions including cough and diarrhea.
Drug Interactions
Passive transfer of antibodies may transiently interfere with the immune responses to live attenuated virus vaccines (eg, measles, mumps, rubella, and varicella).
Please see full Prescribing Information for XEMBIFY.
Important Safety Information
GAMUNEX®-C (immune globulin injection [human], 10% caprylate/chromatography purified) is indicated for the treatment of primary humoral immunodeficiency disease (PIDD) in patients 2 years of age and older, idiopathic thrombocytopenic purpura (ITP) in adults and children, and chronic inflammatory demyelinating polyneuropathy (CIDP) in adults.
Thrombosis may occur with immune globulin products, including GAMUNEX-C. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors. For patients at risk of thrombosis, administer GAMUNEX-C at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur with immune globulin intravenous (IVIG) products in predisposed patients. Patients predisposed to renal dysfunction include those with any degree of preexisting renal insufficiency, diabetes mellitus, age greater than 65, volume depletion, sepsis, paraproteinemia, or patients receiving known nephrotoxic drugs. Renal dysfunction and acute renal failure occur more commonly in patients receiving IVIG products containing sucrose. GAMUNEX-C does not contain sucrose. For patients at risk of renal dysfunction or failure, administer GAMUNEX-C at the minimum concentration available and the minimum infusion rate practicable.
GAMUNEX-C is contraindicated in patients who have had an anaphylactic or severe systemic reaction to the administration of human immune globulin. It is contraindicated in IgA-deficient patients with antibodies against IgA and history of hypersensitivity.
Severe hypersensitivity reactions may occur with IVIG products, including GAMUNEX-C. In case of hypersensitivity, discontinue GAMUNEX-C infusion immediately and institute appropriate treatment.
Monitor renal function, including blood urea nitrogen (BUN), serum creatinine, and urine output in patients at risk of developing acute renal failure.
Hyperproteinemia, increased serum viscosity, and hyponatremia may occur in patients receiving IVIG treatment, including GAMUNEX-C.
There have been reports of aseptic meningitis, hemolytic anemia, and noncardiogenic pulmonary edema (transfusion-related acute lung injury [TRALI]) in patients administered with IVIG, including GAMUNEX-C.
The high-dose regimen (1g/kg x 1-2 days) is not recommended for individuals with expanded fluid volumes or where fluid volume may be a concern.
Because GAMUNEX-C is made from human blood, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
Do not administer GAMUNEX-C subcutaneously in patients with ITP because of the risk of hematoma formation.
Periodic monitoring of renal function and urine output is particularly important in patients judged to be at increased risk of developing acute renal failure. Assess renal function, including measurement of BUN and serum creatinine, before the initial infusion of GAMUNEX-C and at appropriate intervals thereafter.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/markedly high triacylglycerols (triglycerides), or monoclonal gammopathies, because of the potentially increased risk of thrombosis.
If signs and/or symptoms of hemolysis are present after an infusion of GAMUNEX-C, perform appropriate laboratory testing for confirmation.
If TRALI is suspected, perform appropriate tests for the presence of antineutrophil antibodies and anti-HLA antibodies in both the product and patient's serum.
After infusion of IgG, the transitory rise of the various passively transferred antibodies in the patient's blood may yield positive serological testing results, with the potential for misleading interpretation.
In clinical studies, the most common adverse reactions with GAMUNEX-C were headache, pyrexia, hypertension, chills, rash, nausea, arthralgia, and asthenia (in CIDP); cough, rhinitis, pharyngitis, headache, asthma, nausea, fever, diarrhea, and sinusitis with intravenous use (in PIDD) and local infusion-site reactions, fatigue, headache, upper respiratory tract infection, arthralgia, diarrhea, nausea, sinusitis, bronchitis, depression, allergic dermatitis, migraine, myalgia, viral infection, and pyrexia with subcutaneous use (in PIDD); and headache, ecchymosis, vomiting, fever, nausea, rash, abdominal pain, back pain, and dyspepsia (in ITP).
The most serious adverse reactions in clinical studies were pulmonary embolism (PE) in 1 subject with a history of PE (in CIDP), an exacerbation of autoimmune pure red cell aplasia in 1 subject (in PIDD), and myocarditis in 1 subject that occurred 50 days post-study drug infusion and was not considered drug related (in ITP).
Please see full Prescribing Information for GAMUNEX-C.
Terms to know
IgA, immunoglobulin A; IgD, immunoglobulin D; IgE, immunoglobulin E; IgG, immunoglobulin G; IgM, immunoglobulin M; IVIG, intravenous immunoglobulin; PIDD, primary humoral immunodeficiency disease; SCIG, subcutaneous immunoglobulin.
References
GAMUNEX-C Prescribing Information, Grifols. January 2020.
US Food and Drug Administration. July 3, 2019 approval letter - XEMBIFY. Accessed April 25, 2024. https://www.fda.gov/media/128602/download?attachment
US Food and Drug Administration. Gamunex-C safety and utilization review. Accessed April 25, 2024. https://www.fda.gov/media/142078/download
Gelfand EW. Differences between IGIV products: impact on clinical outcome. Int Immunopharmacol. 2006;6(4):592-599.
Jolles S, Orange JS, Gardulf A, et al. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clin Exp Immunol. 2015;179(2):146-160.